Until now, the methods we have discussed for measuring dissolved hydrogen have only focused on measurements using various types of electrical devices. However, we should investigate another simple and proven method of measuring dissolved hydrogen gas using a redox titration reagent.
What is titration?
When chemists are faced with a solution containing an unknown amount of a dissolved compound or gas, a titration is often performed to determine the level of the unknown concentration.
Titration is commonly used to measure such properties as pH, chlorine or dissolved oxygen in water.
A titration involves slowly adding small, precise amounts (drops) of a solution of known concentration (titrant) to a precise amount of a test sample (about 6 mL) containing an unknown concentration of the solute until an indication (such as a color change) is present. that the titration is complete.
This is called the “titration endpoint,” and based on the amount of titrant added to reach the endpoint, the unknown concentration of the solute in the solution can be determined.
About the redox reagent, which is commonly used to detect dissolved hydrogen
When selecting the redox reagent as an indicator for use with a particular solute, care must be taken to ensure that it will react with the solute in question.
Because in our case, since we want to measure dissolved hydrogen gas, a compound is needed that we know will react under the expected conditions of temperature, pH, etc. and will give us visible signs of both the presence of H2 and the achievement of the titration endpoint will provide.
A good choice for measuring dissolved hydrogen is methylene blue (MB), a dark green powder that, like ethanol, turns deep blue when dissolved in a solvent.
MB is used in medicine as a biological tissue dye and agent for the treatment of various diseases.
As an oxidizing agent, MB dissolved in a carrier solvent (in the presence of a platinum catalyst) is known to react with molecular hydrogen.
The end product of the reaction is the clear form of MB, namely leucomethylene blue (LeukoMB), as follows:

As Equation 8 shows, methylene blue (MB) is a blue liquid in its initial state and reacts with H2 to form leucomethylene blue (LeukoMB), the reduced form of MB. Because this form of MB is “clear” when dissolved in water, it meets the requirement that the titrant provide a “visible indication” of the presence of our solute H2.
The two structural diagrams in Figure 15 show both the oxidized and reduced forms of the MB molecule:
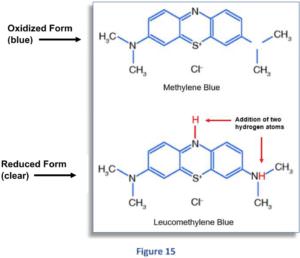
As you can see, the only difference between the two molecules is the addition of the two hydrogen atoms (in red) in the bottom diagram, which are bonded to the MB molecule during the reaction.
These atoms come from the hydrogen molecule, which initially consists of two hydrogen atoms (H2) bonded together.
During the reaction, H2 splits (under the influence of the platinum catalyst) into two hydrogen atoms (H), which then dock onto the MB molecule at two different points.
Therefore, when the methylene blue reagent is added to the sample containing dissolved H2 gas, the H2 molecules are “consumed” when they react with MB. As more drops are added, all of the H2 in the sample will eventually be exhausted. At this point, each drop of titrant added will remain colored blue, indicating that the titration endpoint has been reached. It is also worth noting that there is a one-to-one correspondence in the reaction between the H2 and MB molecules, and each MB molecule consumes one H2 molecule (two hydrogen atoms).
Therefore, knowing the molecular weights of each molecule, it becomes easy to design a reagent that consumes a known amount of dissolved H2 per drop (1 drop typically corresponds to 0,1 mg/L dissolved H2).
This allows us to add the total number of drops required for all of the H2 in the test sample to be consumed (titration endpoint) to determine the concentration of dissolved H2. As with many other redox reactions, the reaction between H2 and MB has low pH sensitivity at the extremes of the pH scale (recommended test sample range is 4 to 10 pH). However, because this method does not rely on the measurement of redox potential, as with an ORP-based device, the pH-sensitive response of the reagent is marginal within the likely pH range of most, if not all, hydrogen drinking water. A popular methylene blue titrant such as H2 blue® offers users a simple, reliable and cost-effective method for accurately determining dissolved hydrogen gas.
Excerpt from the book by Randy Sharpe: “The relationship between dissolved H2, pH and redox potential”




