John R.: How long can I drink the alkaline active water? How long is it active? When does it lose its usefulness?
This question concerns the duration of the relaxation time, which can be considered the core term for electroactivated water. This is about the period in which alkaline activated water retains its antioxidant properties. After the relaxation time has elapsed, it is only alkaline water, no longer active water.
Going back to the researchers Prilutsky and Bakhir (Electrochemically activated water: anomalous properties, mechanism of biological action, Moscow 1997), relaxation time was understood as the period in which an exceptionally low redox potential can be measured in basic activated water. This varies from place to place, from water to water, in every climatic situation. It is therefore difficult to predict. Ultimately, there is no avoiding empirical measurement.
Compared to acidic activated water, which lasts for years under favorable circumstances, alkaline activated water has a very short relaxation time of a few minutes to a few days. This is a so-called metastable state. Hydroxide ions and hydrogen content contribute directly to this index parameter. The type and amount of cations also play a role. (See also –> Redox value of activated water).
The most volatile parameters are the H atoms formed on the cathode, whose antioxidant ability can be demonstrated, for example, by the reduction of tungsten trioxide. Hydrogen atoms combine very quickly to form H2 – molecular hydrogen – hydrogen gas. Both can have antioxidant effects. How? –> Redox value of activated water
Since 1997, Sanetaka Shirahata (Shirahata et. al., Electrolyzed reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem. Biophys. Res. Commun., 234, 269174, 1997. ) has also discovered atomic hydrogen permanently in activated water and demonstrated that it protects against oxidation by free radicals at the DNA level, various hypotheses have emerged about where and for how long these hydrogen atoms “park” before combining to form hydrogen gas. Dietmar Ferger, for example, represents the hypothesis of the so-called basic nano-mineral colloids, which has neither been refuted nor proven. Quote: Ferger, Jungbrunnenwasser, Weil am Rhein, 2011, p. 71:
“In effect, an 'electron cloud' is created that surrounds basic minerals and hydrogen and binds them together. In this way, the hydrogen is also negatively charged and activated, creating the so-called >>Active Hydrogen<<." It is doubtful whether the latter, rather borderline scientific explanations for the behavior of alkaline activated water are actually correct and even necessary. In my opinion, the antioxidant properties of water that is only saturated with hydrogen gas are sufficient to explain the phenomena. It is quite clear that hydrogen saturation is the main responsible for the negative redox potential. If the hydrogen content, which is somewhat more difficult to measure, increases, the redox potential (ORP) also decreases. However, this relationship is not proportional, so measuring the redox potential does not provide any information about the amount of dissolved hydrogen. In continuous ionizers, where the water is ionized in a pressure-tight electrolysis cell, an excess pressure of hydrogen gas is created in the cathode chamber, as under normal conditions only dissolve a maximum of 1500 micrograms/l of hydrogen gas in water, although significantly more is produced during electrolysis. Therefore, when emerging from the spout of a water ionizer, hydrogen gas bubbles form, which are released into the atmosphere after a few seconds unless they are drunk with the completely fresh, still bubbling alkaline activated water. With a not With a pressure-tight top ionizer, activated water completely saturated with hydrogen gas can be created in the cathode chamber.
However, the formation of bubbles and outgassing of the excess occurs during the longer electrolysis process, in which the water is also heated, which significantly reduces the hydrogen content. The rule applies: the cooler, the better. But not colder than 40 C.
Both with one pressure-tight Using a top ionizer and a modern 9-electrode flow device, I was able to produce alkaline activated water with complete hydrogen saturation and also supersaturated water with up to 1800 micrograms/l, which, however, returns to normal saturation within minutes.
Since Shigeo Ohta's research began in 2007, there can hardly be any doubt that hydrogen gas (H2) plays a decisive role in the antioxidant performance of alkaline activated water. (Overview: Ohta, S., Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications, Methods Enzymol. 2015;555:289-317).
It is therefore important to design a water ionizer in such a way that as much hydrogen gas as possible is dissolved in the water at a pH value of 8,5 to 9,5, which is ideal for drinking.
Compared to the model from Nihon Trim, which Shirahata used in 1997 and thus achieved a hydrogen content of only between 200 and 350 micrograms/l in the drinking pH range, significant performance increases of more than 2010 times were achieved between 2015 and 5. Other new technologies have already achieved complete hydrogen saturation of 1500 micrograms in the prototype. For further information on hydrogen saturation, please read the FAQ Hydrogen Rich Water.
However, extending the relaxation time by preventing the outgassing of hydrogen is of crucial importance. Because you can't always drink the freshly ionized alkaline active water straight away. There is a clear advantage here in the combination of very dense materials such as stainless steel and thick blue glass with cool horizontal storage when the bottle is completely filled without any air bubbles. We tested the following materials and measured them again after 19 hours of horizontal storage (except the crystal carafe) in the refrigerator:
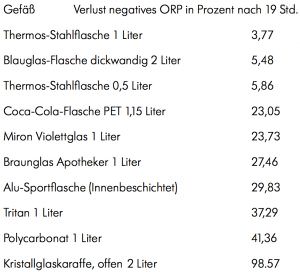
Back to your question and my experience after thousands of measurements of redox potentials:
Greatest benefit when drinking immediately. Great benefit within the first 3 hours. High benefit up to 36 hours. Good benefit up to 48 hours. Afterwards, the water has usually normalized electrochemically, the ionized mineral excess has visibly precipitated and the water is softer.
It's still usable drinking water, but you should use it for tea or for watering flowers.
Excerpt from the book by Karl Heinz Asenbaum: “Electro-activated water – An invention with extraordinary potential. Water ionizers from A – Z”
Copyright 2016 www.euromultimedia.de




