We have shown that the negative ORP value cannot be directly correlated with a specific dissolved H2 content. But is it possible to use the ORP measurements of two different water samples to compare the relative amounts of dissolved H2 contained in each of those water samples? Let's look at an example that shows why the ORP measurement cannot be used to compare dissolved H2 levels.
While the logical assumption might be that the water with the more negative ORP will also contain the highest level of dissolved H2, the following example shows why this assumption is not necessarily true.
Table 3 shows the predicted ORP for two hydrogen waters A and B with different pH and dissolved H2 values:
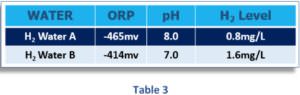
Water A with a pH of 8 and an H2 content of 0,8 mg/l shows an ORP value of -465 mV. But Water B, with a pH of 7,0 and an H2 content of 1,6 mg/L (twice as much H2 as Water A), only measures an ORP of -414 mV. Since the pH of A is one pH unit higher than that of water B, its higher pH produces a more negative ORP even though B has more dissolved H2. This scenario shows why it is wrong to assume that water with a more negative ORP value has more dissolved H2.
Some vendors use the technique of slowing an ionizer's water flow to a "trickle" before measuring the ORP. This practice usually makes the ORP more negative, but mainly because it increases the pH.
The more negative ORP does not necessarily indicate the presence of more dissolved H2; in fact, the water with the more negative ORP could easily contain less H2.
Excerpt from the book by Randy Sharpe: “The relationship between dissolved H2, pH and redox potential”




