The Nernst equation, named after the German chemist Walther Nernst, is an equation that relates the standard reduction potential of an electrochemical reaction to the concentration, pressure and temperature of a chemical species that is reduced and oxidized under non-equilibrium conditions. It is the most important equation in the field of electrochemistry.
Equation 5 shows the general form of the Nernst equation. In this article, we will use the Nernst equation to analyze the roles of dissolved hydrogen gas and pH 5 to predict how much each influences the ORP measurement. Before we can use the Nernst equation to predict ORP values for water with dissolved hydrogen gas, we must slightly modify its general form.
Since the standard cell potential for hydrogen is E0 = 0 (see Table 1), we can eliminate this expression. We will also replace the terms “[red]” and “[ox]” with the reduced and oxidized forms of hydrogen H2 and H+, respectively.
The final form of the Nernst equation that we will use is shown in Equation 6:


Since the hydrogen water we measured does not exist under standard conditions, the Nernst equation allows us to substitute any concentration, pressure and temperature values we choose and predict the redox potential under non-standard conditions.
Using the Nernst equation, we can experiment with different values for H2 and H+ and analyze their predicted effect on ORP. Below are definitions for the terms and values used in the Nernst equation:
- Emv, Nernst potential in millivolts (ORP);
- E0, standard cell potential, 0,00 V;
- R, universal gas constant, 8,314 JK-1mol-1
- T, temperature, 298,150 K (25 °C / 77 °F);
- z, # number of electrons transferred in the reaction, 2;
- F, Faraday constant, 96485,33 Cmol-1 (electron charge per mole)
- [H2], hydrogen gas concentration (at partial pressure, pH2);
- [H+], hydrogen ion concentration (derived from pH).
ORP values predicted by the Nernst equation are calculated under ideal conditions. While actual field measurements of ORP vary depending on a number of factors, the relationships between pH, H2 and ORP remain the same.
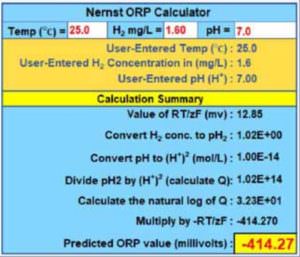
To automate the calculation of the predicted redox potential under different temperature/H2/pH scenarios, the Nernst equation was programmed into a calculator (using MS-Excel/VBA).
The calculated results for each scenario were then used to create the H2, pH and redox data table (with pairs ordered by x and y) required to plot the various graphs used in this article.
Figure 4 shows the user interface of the Nernst calculator, which displays not only the predicted redox potential, but also most of the intermediate results:
Although it is not our goal to discuss the Nernst equation in detail, a closer look at Equation 6 will give us important insight into the calculation of the ORP. Figure 5 shows the two species from the Nernst equation that form our “redox couple of interest”:
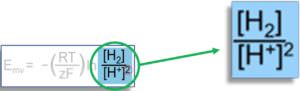
As we previously discussed, these two forms of hydrogen (oxidized and reduced forms) represent the two species in water whose concentrations contribute to the redox potential of water. While water normally contains several redox couples whose redox potentials together form the total ORP, in our analysis we only consider the H+/H2 redox couple. The expression “[H2]” represents the concentration of dissolved hydrogen gas (expressed as partial pressure, pH2) and the expression “[H+]” represents the concentration of hydrogen ions (which we calculate from the pH of the water).
Therefore, the ORP reading does not depend on the concentration of dissolved hydrogen gas alone, but on the concentrations of both H2 and H+.
As we will see, each of these species contributes to the ORP measurement. Since we have seen that solved H2 is responsible for the negative ORP, this implies a relationship between the two and raises some questions:
- How much dissolved H2 is needed to produce a negative ORP?
- How strongly does the ORP respond to changes in H2 concentration?
- Can we use ORP value to measure dissolved H2 content?
- Can we use the ORP measurements from two different samples to compare their relative dissolved H2 concentrations?
The Nernst equation will help us answer these questions because it allows us to analyze how changes in H+ and H2 affect the ORP measurement.
Excerpt from the book by Randy Sharpe: “The relationship between dissolved H2, pH and redox potential”




